Tigilanol tiglate: Human oncology
Our lead molecule, tigilanol tiglate, is a novel small molecule administered directly into a solid tumour mass. A single intratumoural injection of tigilanol tiglate rapidly destroys injected tumours, with minimal tumour recurrence.
Tigilanol tiglate acts directly via disrupting the tumour blood vessels that supply the tumour, and by directly killing tumour cells through a process called oncosis. Tigilanol tiglate also induces rapid healing of the tumour site. Non-injected tumours may also be targeted by indirect systemic anti-tumour responses (Fig 1).

Source: Biopharma Dealmakers (Biopharm Deal) ISSN 2730-6283 (online)
Fig. 1 | Intratumoural treatment with tigilanol tiglate induces injected and systemic non-injected tumour responses.
(a) Injected tumours are rapidly destroyed by tumour cell necrosis and tumour vascular disruption. (b) The disrupted tumours release tumour-derived antigens which generate systemic anti-tumour responses against non-injected neighbouring or distant tumours. DAMPs, damage-associated molecular patterns.
We have clinical and pre-clinical evidence that tigilanol tiglate has “pan-tumour activity” with the potential to treat a broad range of solid tumours. While effective as a single agent therapy, it also induces synergistic anti-tumour responses when combined with other cancer therapies, including immune checkpoint inhibitors, chemotherapy and radiotherapy.
Tigilanol tiglate has the potential for improved efficacy over other intratumoural treatments. It can completely destroy the injected tumour with a single treatment, and without damaging normal tissue. Therefore, it may significantly improve outcomes for patients with unresectable tumours, tumours in locations that are difficult to access, or where surgery could affect patients’ ability to hear, see, smell, swallow or taste such as in head and neck cancer.
Strong efficacy signals from a first-in-human, dose-escalation safety trial
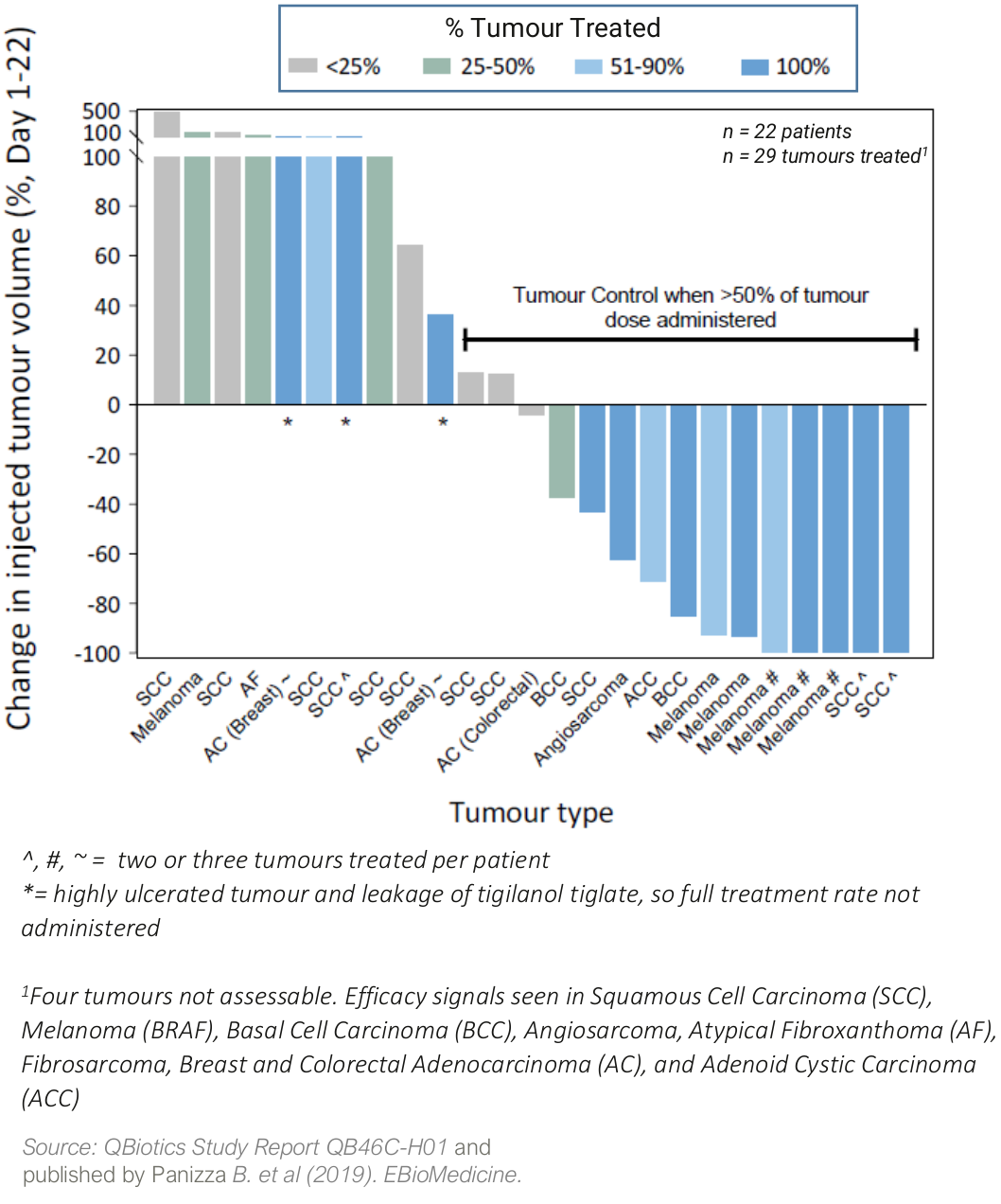
A dose-escalation safety Phase 1 trial in 22 patients with a broad range of cutaneous and subcutaneous solid tumours1 showed:
- tigilanol tiglate was generally well tolerated with a maximum tolerated dose (MTD) not declared;
- injected tumour control rate (CR/PR/SD)* of 60% from a single injection
- best Target Tumour Response (RECIST 1.1; Day 1-22)
- four patients (18%) achieved a Complete Response (CR, full tumour destruction)
- five patients (22%) achieved a partial response (PR or >30% tumour reduction)
- fourteen patients (64%) had stable disease (SD)
* CR= Complete Response; PR = Partial Response or >30% tumour reduction; SD = Stable Disease
Two patients with metastatic melanoma also had distal tumour responses, so-called abscopal or anenestic responses, where their non-injected tumours also responded to tigilanol tiglate treatment.
Tumour response of single IT treatment at escalating doses
Although Phase 1 trials are not powered for efficacy, the strong tumour responses observed in the first human study is supported by impressive efficacy data seen in dogs. In dogs, tigilanol tiglate is approved as a first-line alternative to surgery in non-metastatic mast-cell tumours, meeting an unmet need in the veterinary market.
In a pivotal multicentre study in 123 canine patients with mast-cell tumours, a single injection induced a 75% Complete Response at 28 days post-treatment, with an Objective Tumour Response of 80%. Two injections led to an 88% Complete Response. One year later, 89% of the dogs had no tumour recurrence.
These spontaneous tumours in animals provide a clinically relevant and highly validated model for cancer in humans.
- Panizza et al. (2019). EBioMedicine, 9(41), 1-9.
